CRISPR
CRISPR-based Somatic Engineered Murine Models (SEMMs) for Translational Research
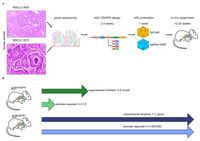
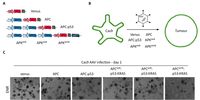
Lung cancer is the most common cancer worldwide and the leading cause of cancer-related deaths in both men and women. Despite the development of novel therapeutic interventions, the 5-year survival rate for non-small cell lung cancer (NSCLC) patients remains low, demonstrating the necessity for novel treatments. One strategy to improve translational research is the development of surrogate models reflecting somatic mutations identified in lung cancer patients as these impact treatment responses. With the advent of CRISPR-mediated genome editing, gene deletion as well as site-directed integration of point mutations enabled us to model human malignancies in more detail than ever before. Here, we report that by using CRISPR/Cas9-mediated targeting of Trp53 and KRas, we recapitulated the classic murine NSCLC model Trp53fl/fl:lsl-KRasG12D/wt. Developing tumours were indistinguishable from Trp53fl/fl:lsl-KRasG12D/wt-derived tumours with regard to morphology, marker expression, and transcriptional profiles. We demonstrate the applicability of CRISPR for tumour modelling in vivo and ameliorating the need to use conventional genetically engineered mouse models. Furthermore, tumour onset was not only achieved in constitutive Cas9 expression but also in wild-type animals via infection of lung epithelial cells with two discrete AAVs encoding different parts of the CRISPR machinery. While conventional mouse models require extensive husbandry to integrate new genetic features allowing for gene targeting, basic molecular methods suffice to inflict the desired genetic alterations in vivo. Utilizing the CRISPR toolbox, in vivo cancer research and modelling is rapidly evolving and enables researchers to swiftly develop new, clinically relevant surrogate models for translational research.
Organoids
Patient-derived and murine organoid models of lung and intestine
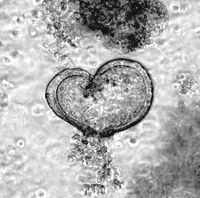
Long term culture of tracheal and alveolar organoids is feasible for up to 6 months. Immunohistologic analysis of murine tracheal organoids revealed that keratinisation is maintained (KRT5+), and basal cells (Sox2+) are not lost during long term culture . As basal cells are believed to be one ‘cell of origin’ for SCC, we genetically manipulate these organoids by CRISPR/Cas9 gene editing to mutate or overexpress classic SCC oncogenes, and co-deplete commonly lost tumour suppressors, such as TP53 or STK11/LKB1 . Alveolar organoids, which are comprised of AT-1 (Pdpn+) and AT-II cels (SftpC+), are utilized to model ADC, as in vivo studies had identified the AT-II as well as bronchio-alveolar ducts junction cells (BADJ) as 'cell of origin' for NSCLC ADC. Apart from genetically tailored murine respiratory organoid models do we culture and propagate patient organoids, derived from resection material. To identify the genetic alterations within these primary human explant, panel sequencing is conducted to delineate the underlying genetic alterations. Combining genetically modified organoids with patient organoids, together with the corresponding control organoids, allows us the testing novel therapeutic strategies for the treatment of NSCLC-ADC and -SCC.
Intestinal organoids are an ideal system to reconstruct the typical crypt-villus structure of the intestine. These so-called mini-intestines grow in 3D and consist of all cell types found in the gastrointestinal tract. In our laboratory, we have produced both murine and patient-derived intestinal organoids from transformed as well as untransformed tissues. By using constitutive Cas9-expressing murine small intestinal organoids, we have introduced various mutations that occur in colorectal cancer patients to recapitulate all stages of colorectal cancer and to study the changes in proteins of interest during tumorigenesis. Using the high-content imaging facility, we can analyse changes in the morphology, stem cell composition, and proliferation of these organoids.